Essure A Contraceptive Device in Debate
Introduction
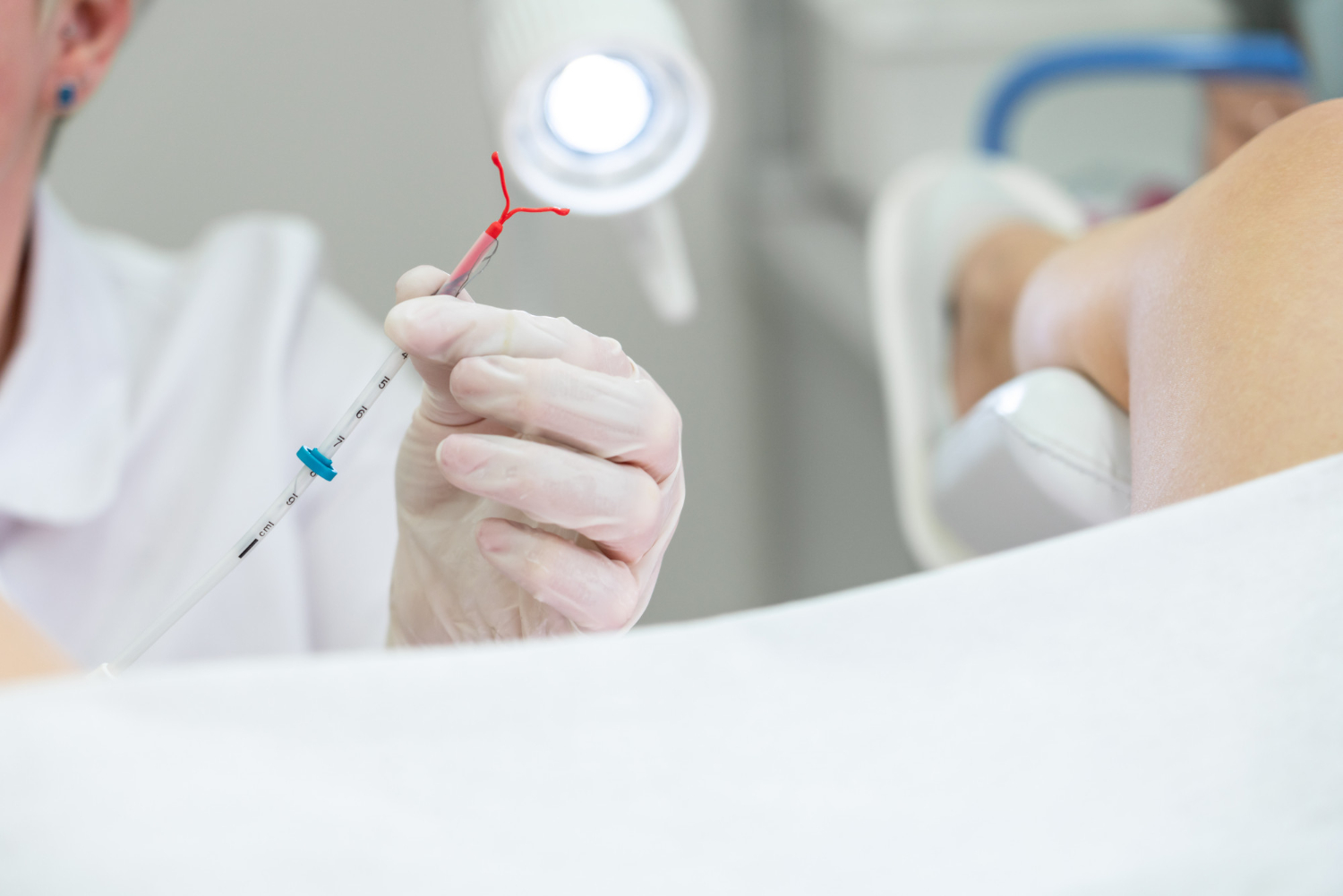
When a product promises the sky but fails to deliver, one anticipates a legal battle between its users and the manufacturer/s; Essure has met with a similar fate. Essure® is an intrauterine device developed by Conceptus Inc., a subsidiary of Bayer AG, and approved by the FDA in 2002. It was marketed as a permanent surgical sterilization option for women. This device is composed of two metal coils made from polyester fibers, nickel-titanium, stainless steel, and solder that are placed in the fallopian tubes to induce fibrosis and block them to prevent fertilization.
Essure brought with it many promises: a perfect option for women looking out for permanent surgical sterilization and a perfect alternative to the then-existing tedious tubal ligation procedure. The timing couldn't have been better. Essure qualified for all the 4 P's of marketing - Product, Price, Placement, and Promotion. It entered a ripe market that was willing more than ever to use this new product. It wasn't surprising to know that Essure became the market leader, gaining prescriptions, in thousands, within a short span of time.
Food and Drug Administration's (FDA) Take On The Controversial Contraceptive Device
- In September 2015, the FDA held a meeting to examine the safety of the product after receiving thousands of adverse event reports.
- On October 31, 2016, the FDA issued final guidance, “Labeling for Permanent Hysteroscopically-Placed Tubal Implants Intended for Sterilization,” after carefully considering public comments.
- On November 11, 2016, FDA announced label changes adding a boxed warning and a Patient Decision Checklist. This was aimed to support patient counseling and understanding of risks and benefits associated with Essure, as well as what to expect during and after the Essure procedure.
- On April 9, 2018, FDA restricted the sale of Essure devices to only those doctors and healthcare facilities who use the FDA-approved “Patient-Doctor Discussion Checklist – Acceptance of Risk and Informed Decision Acknowledgement.” In July 2018, Bayer announced Essure would be wiped out of the market by December 31, 2018.
- In January 2020, the FDA said that all the Essure devices were removed from the market, and the women who had permanently implanted the device were enrolled in a study to evaluate the risk associated with it.
- In July 2020, the FDA's ongoing study over Essure indicated that more than 20% of women implanted with the birth control device might be suffering from allergic or hypersensitivity reactions.
However, this rise was followed by a fall; the sales figures started plunging. Between 2002 and 2015, the company, itself, received more than 30,000 adverse event reports. No initiative was shown to resolve these. The list included persistent pain, perforation of the uterus and/or fallopian tubes, intra-abdominal or pelvic device migration, abnormal or irregular bleeding, and allergy or hypersensitivity reactions, including reports of unintended pregnancies.
Despite such grave complaints, the product wasn't withdrawn from the market. Subsequently, the FDA passed an order for the product package to carry a 'black box' warning, a patient checklist to be completed by the patient, and also ordered Bayer to conduct post-market surveillance.
Other Contraceptive Devices in Debate
Mirena is a type of long-acting, reversible hormonal intrauterine device (IUD) manufactured by Bayer Pharmaceuticals. It is a small T-shaped flexible device that is placed into the uterus by a trained healthcare provider during a routine office visit. Once implanted, Mirena releases small amounts of a progestin hormone called Levonorgestrel into the uterus. It provides continuous, highly effective birth control.
There were incidences where the device got migrated outside the uterus or got lodged in the uterine wall, some females conceived in spite of the device placement and landed up in undue complications. All these pieces of evidence brought the Mirena device in trouble.
ParaGard, a small IUD (intrauterine device) that is used to prevent pregnancy, works differently using one simple active ingredient, copper, instead of hormones, unlike its other counterparts. It is a T shaped device and can help avoid pregnancy for up to 10 years.
Women who were implanted with the device have claimed that the device fractured or broke during removal, which resulted in injuries and painful complications.
Aggrieved women have taken Bayer to the court; lawsuits were filed across the country. To prevent duplicity and inconsistent ruling, Judge Smith from California, consolidated 11 Essure lawsuits in August 2016 as they had common factual and legal questions. The judge ordered these cases to proceed on the liability issue. This was followed by the coordination of 55 more cases in November 2016. The ruling of the first case will be a turning point for hundreds of pending Essure cases. The long wait may end a time; one verdict will change the fate of thousands. Be ready for the high tide of cases to hit you. Get a quality Medical Record Review for Essure Litigations at a flat rate of $100 per review from Neural IT to plug every loophole and make it a watertight case. We invite you to benefit from our record review services.
For more information, on other Mass Torts please click here. You may email us at or call +1-844-NIT-TEAM (648-8326).




