16 Zofran Lawsuits to be Picked for Early Bellwether Trials
16 Zofran Lawsuits to be Picked for Early Bellwether Trials
Introduction
On May 17, 2018, U.S. District Judge Dennis Saylor ordered both parties involved in the Zofran litigation over birth defects to pick a total of 16 cases to proceed to early bellwether trials, assigning half to each side. This would help evaluate the reaction of the jury to certain evidence and testimony. As per the order, the parties have time until June 15 to select the cases.
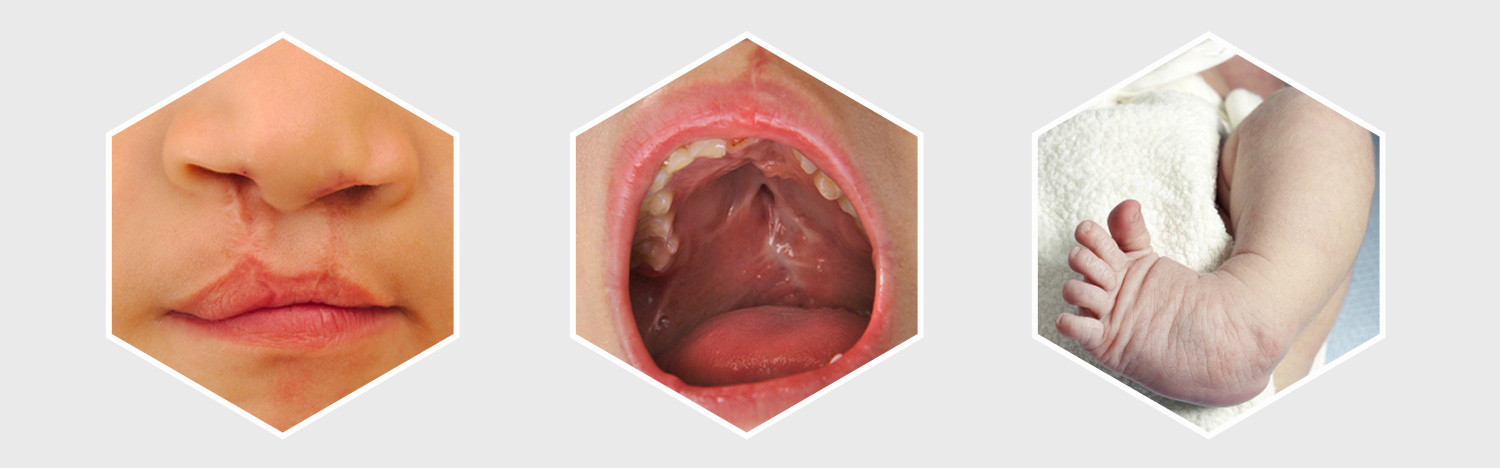
The order stated that it was mandatory for the cases to include completed fact sheet and all relevant authorizations details submitted to GlaxoSmithKline at least 60 days in advance of case selections. The claims must cover either structural heart damage birth defects, like atrial or ventricular septal defects, or craniofacial birth defects, such as cleft palate and cleft lip. After the lawsuits are selected for bellwether trials, the parties have time till October 31 to discuss the procedures to choose individual cases from that group of lawsuits for future trials.
At present, more than 450 product liability cases are filed against GlaxoSmithKline, over failure to provide timely warning to doctors about the adverse effects of Zofran consumption during pregnancy. The drug was FDA approved to treat nausea in cancer patients, but GlaxoSmithKline took the liberty of marketing it to pregnant women for morning sickness. In October 2015, a multidistrict litigation [MDL No. 2657 In Re: Zofran (Ondansetron) Products Liability Litigation] was created for Zofran birth defects lawsuits in the District of Massachusetts and is presided by Judge Dennis F. Saylor, IV.
In another recent event, GlaxoSmithKline succeeded in getting the federal judge to toss the lawsuits filed by three women over injuries allegedly linked to the generic form of Zofran (also developed by GlaxoSmithKline LLC) from the Zofran multidistrict litigation.




