Biomet's Motion For Mistrial Over Punitive Damages Denied
Biomet's Motion For Mistrial Over Punitive Damages Denied
Introduction
On Wednesday, U.S. District Judge Stephen Clark of the U.S. District Court for the Eastern District of Missouri rejected Biomet Inc.'s motion for mistrial in the punitive damages phase of an M2a Magnum hip implant lawsuit, which the manufacturer had pushed for reconsideration after one juror contracted COVID-19.
According to the court documents, the punitive damages phase concluded the same day. However, the verdict was not made publicly available.
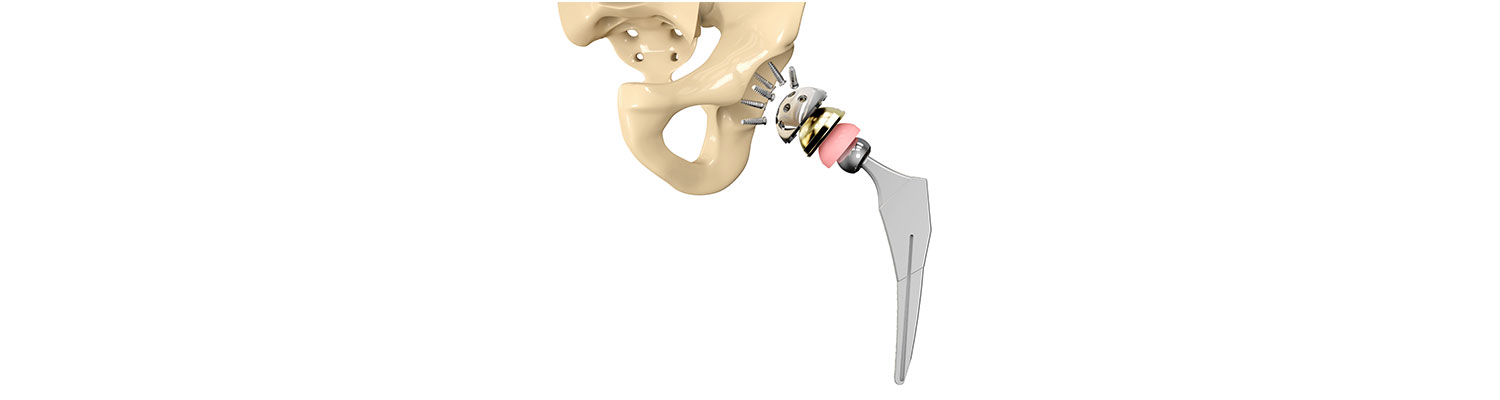
The plaintiff involved in the lawsuit had both her hips replaced with Biomet's M2a Magnum in early 2008. The plaintiff started experiencing pain in her left hip in 2010, which resulted in her first revision surgery in March 2011. A lawsuit was filed in 2013, alleging that the hip implant was defective and the manufacturer should have known about it as the design was based on an earlier design, called the M2a Taper, which had allegedly caused problems previously.
The lawsuit went to trial last month, and a compensatory damages verdict was concluded in favor of the plaintiff on October 22.
Following the verdict, attorneys representing the defendant asked the court to reconsider the compensatory-damages verdict, stating that one juror tested positive for the COVID-19 test during the earlier phase. The defendant again pushed for a mistrial on Wednesday, arguing that trying the second phase to a different jury after the removal of one juror violated the 7th Amendment rights.
The federal judge ruled against the defendant's motion for a continuance or mistrial, and the reasoning of the order was not promptly clear.
Zimmer Holdings is an Indiana-based orthopedics company involved in providing hip and knee solutions including joint and skeletal replacement systems and surgical tools as well as biologics designed to assist in joint replacement surgeries. In April 2014, Zimmer and Biomet announced to come together to provide joint replacement, bone repair, and dental implant solutions. In June 2015, they started out as Zimmer Biomet and began offering solutions to the musculoskeletal healthcare industry. The hip and knee implants were intended to relieve pain, provide good quality of life to the patients. However, Zimmer lawsuits filed by several individuals claimed the metal-on-metal articulation used in the system resulted in Metallosis, because of the metal grounding on metal as it leads to toxic metal shards being released into the patient's surrounding tissues and bone




