FDA Warns of High Fracture Risk With Zimmer Biomet Hip Device
FDA Warns of High Fracture Risk With Zimmer Biomet Hip Device
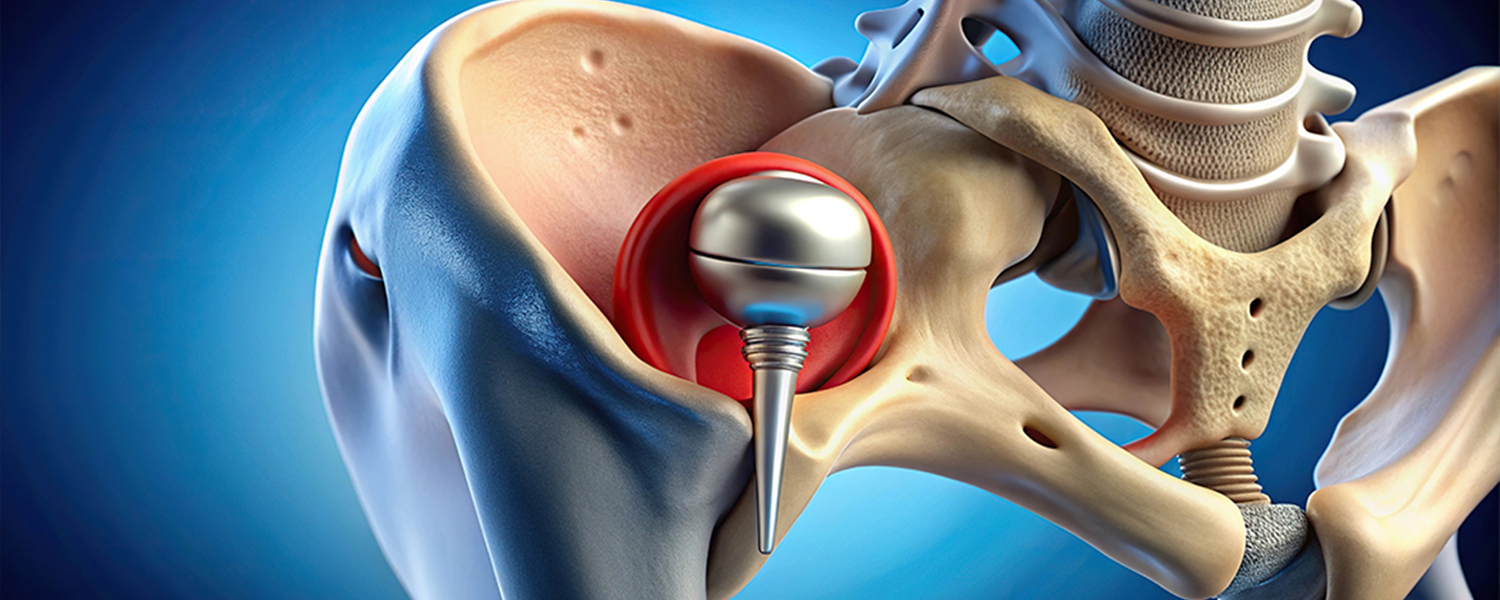
Introduction
The U.S. Food and Drug Administration (FDA) has issued a safety alert regarding an increased risk of thigh bone fractures associated with the Zimmer Biomet CPT Hip System, which had previously been recalled.
FDA advises patients, caregivers, healthcare providers, and facilities to consider alternative prostheses when possible. If the use of the CPT Hip System is necessary, patients should be informed of the elevated risk.
The CPT Hip System Femoral Stem 12/14 Neck Taper, a polished-taper slip (PTS) style stem made from cobalt chromium alloy, is commonly used in hip replacement surgeries. However, recent studies have revealed a higher likelihood of postoperative periprosthetic femoral fractures (thigh bone fractures after surgery) with this device compared to similar hip prostheses.
 In early July, Zimmer Biomet recalled the CPT Hip System to update its instructions for use due to the increased fracture risk and announced plans to phase out the product by December 2024. Despite this, the FDA has expressed concerns about the continued use of the system for new patients, given the elevated risk of fractures and the potential need for additional surgery if a fracture occurs.
In early July, Zimmer Biomet recalled the CPT Hip System to update its instructions for use due to the increased fracture risk and announced plans to phase out the product by December 2024. Despite this, the FDA has expressed concerns about the continued use of the system for new patients, given the elevated risk of fractures and the potential need for additional surgery if a fracture occurs.
The FDA is working with Zimmer Biomet to address these concerns and ensure that all parties involved—patients, caregivers, and healthcare professionals—are aware of the risks associated with the CPT Hip System. The agency will continue to monitor the situation to help minimize the risk of injury and ensure patient safety.




