Federal Judge May Include Smith & Nephew Hip Replacement Lawsuits with Hip Resurfacing MDL
Federal Judge May Include Smith & Nephew Hip Replacement Lawsuits with Hip Resurfacing MDL
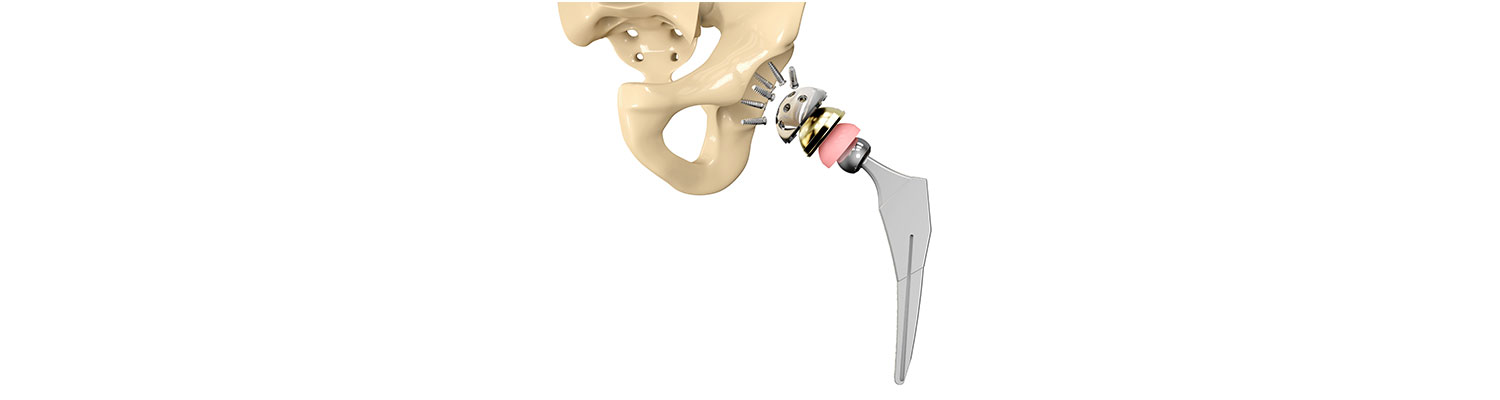
Introduction
Federally-filed Smith & Nephew Birmingham Hip Replacement litigation is witnessing a steady growth in size with additional cases being filed by plaintiffs. As of April, there were more than 250 cases filed as a part of this litigation. Allegations include that the metal-on-metal hip resurfacing and replacement designs are defective and prone to fail, often requiring revision surgery to have them removed and replaced. The U.S. JPML decided to consolidate these cases in Maryland under Judge Catherine C. Blake in May 2017.
In a case management order (PDF) released by Judge Blake on May 3, both the parties agreed to include all the component related complaints in the Hip resurfacing system litigation, even though the component was used as part of a total hip replacement. As per the order, the hip replacement lawsuits will proceed on a separate track from the claims involving hip resurfacing procedures, which would be identified by the court periodically.
Hip replacement lawsuits continue to be filed under a related hip litigation involving Stryker Hip Lawsuits, centralized before U.S. District Judge Indira Talwani under MDL No. 2768 – in re: Stryker LFIT V40 Femoral Head Products Liability Litigation.




