New Study Strengthens Elmiron And Vision Problems Risks
New Study Strengthens Elmiron And Vision Problems Risks
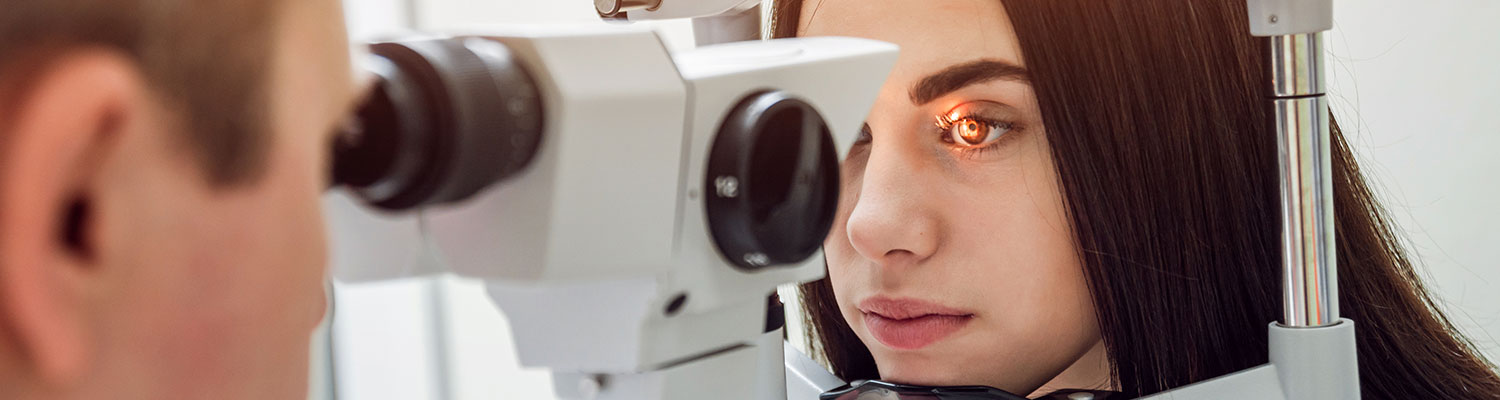
Introduction
On February 11, a new study was published in the medical journal Clinical Ophthalmology in which Northwestern University researchers provided stronger evidence that supports the link between Elmiron (pentosan polysulfate sodium or PPS) and the related cases of retinal maculopathy.
The study was conducted at the Northwestern Ophthalmology clinic, where the researchers reviewed the data of 131 Elmiron users. 40 patients from the total had undergone imaging and 5 of them had features believed to be of Elmiron maculopathy. Similarly, 5 patients from the remaining 91 patients also showed features of macular pigmentary changes.
The ten patients out of the total who showed signs of maculopathy had used Elmiron for 4.2 years on average with a cumulative dose of 380g. The median cumulative dose for the non-suspect group was only 188.1g, as compared to an average of 317g among the suspect group.
Thus, the new study indicates that Elmiron-related maculopathy has a distinct signature, which can be identified using multimodal imaging.
Currently, more than 100 product liability lawsuits are pending in the U.S. against the manufacturer of Elmiron, Johnson & Johnson's (J&J) subsidiary Janssen Pharmaceuticals, Inc., each claiming that its users suffered severe retina damage and permanent vision problems, including difficulty adapting to the dark light, spots, or floaters in the vision and complete blindness.
Elmiron lawsuits are consolidated under MDL 2973, which is presided by U.S. District Judge Brian R. Martinotti in the United States District Court for the District of New Jersey.
The litigation seems to be accelerating as the presiding judge appointed a 25-member leadership committee last month. The committee consists of three Co-lead Counsel and one Liaison Counsel, seven Executive Committee members, and 14 Steering Committee members.




