Zimmer Biomet Asks To Toss $3.55M Hip Implant Verdict
Zimmer Biomet Asks To Toss $3.55M Hip Implant Verdict
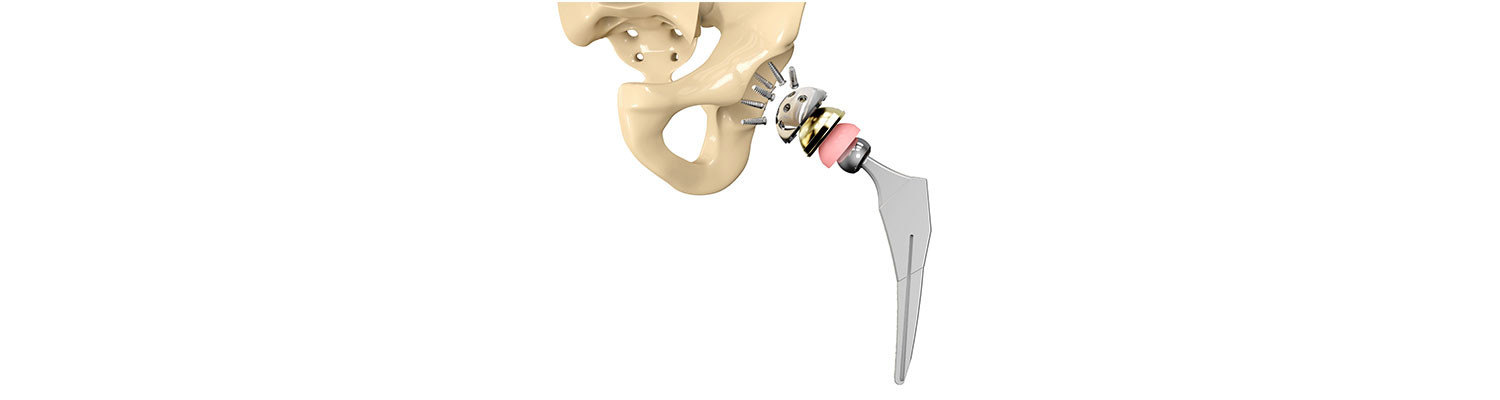
Introduction
Last Friday, Biomet Inc. filed a pair of briefs seeking a judgment or a new trial over a federal jury’s $3.55 million judgment in a lawsuit brought by a couple alleging the company's metal-on-metal hip implant.
According to the briefs filed, the company argued over the couple's failure to present a piece of evidence that shows the M2a implant caused injuries to the wife.
The company also attacked the evidence submitted by the couple, saying that the court prejudiced the company by allowing the plaintiffs to submit a slew of evidence and stopping the company from presenting evidence in rebuttal.
The initial verdict was awarded by an Iowa federal jury over the claims that the Biomet M2a Magnum metal hip dispersed microscopic particles and resulted in revision surgery. The couple was awarded $1.05 million in compensatory damages and $2.5 million in punitive damages.
The company also found fault with the jury instructions given by the court, stating that it did not take into account the warnings placed on the products, which it believes is adequate as a matter of law.
The company calls the verdict excessive and a miscarriage of justice, arguing that the errors made by the court and the insufficiency of the evidence demand either a judgment in the company's favor or a new trial.
In December, Biomet had filed a motion in the U.S. District Court for the Eastern District of Missouri, asking the federal judge to overturn the verdict over a case, considered "virtually identical" to this trial, that concluded in Missouri, where the state's federal jury awarded $21 million to a plaintiff and her husband.




