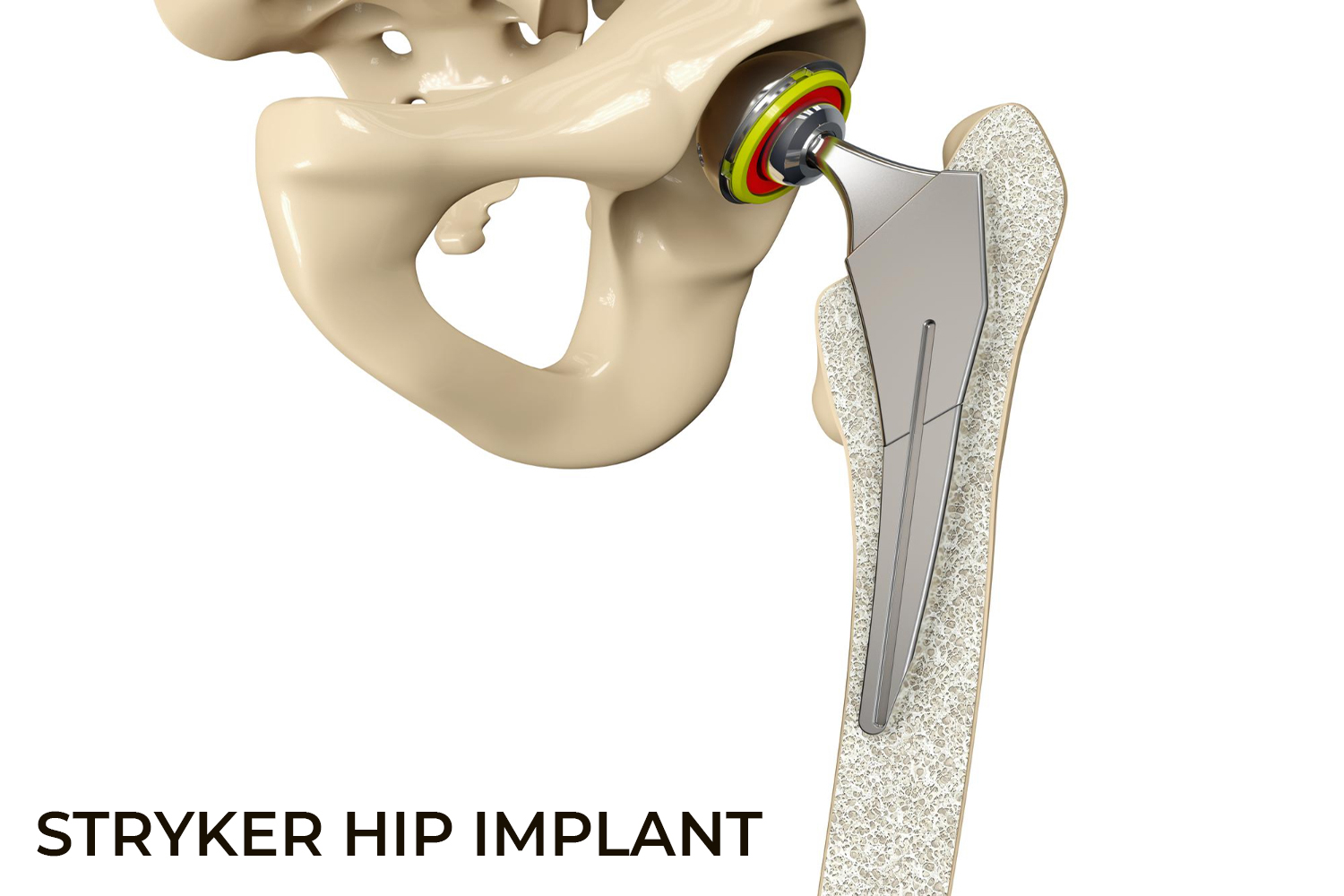
Stryker Orthopaedics is known for the development and manufacture of specialty surgical products and other medical supplies for health care around the world. The company has manufactured scores of medical devices and joint replacement products, most of which have been successful; however, some of the hip replacement devices have caused serious adverse events, injuring hundreds of patients.
The Stryker Rejuvenate Modular-Neck Stem and the ABG Modular-Neck Stem hip replacement devices were approved by the Food and Drug Administration (FDA) in 2008 and 2009. These devices are not typical "metal-on-metal" (MoM) as the necks are built of Cobalt and Chromium and the neck stems are layered with Titanium. Though the intention was to make it corrosion resistant, the two metals rubbed on each other at the connection and released toxic metal ions and debris. Stryker permanently recalled the Rejuvenate Modular and ABG II Modular-Neck Hip Stems in 2012 and stopped all global sales and production of these components. Another hip stem model, the Accolade TMZF, also caused problems and was recalled in 2009, 2011, and 2013.
Most patients with Stryker Rejuvenate and ABG II implants have undergone additional surgery or revision surgeries to remove and replace the implant to repair or reconstruct injured joints, bones, and tissues in addition to the replacement surgery.
Michigan-based Stryker Corporation began as the Orthopedic Frame Company in the 1940s. Over the years the company expanded and now markets 57,000 products worldwide. Its net sales reached $9.9 billion in 2015, and 13 percent of those sales came from hip replacement products, according to the company's annual report.
The company introduced its newest hip replacement product, the ADM X3 Mobile Bearing Acetabular System, in 2010. The ADM X3 is an acetabular component that uses a dual mobility cup concept that has been proven to reduce the risk of dislocation and improve stability. It's made from polyethylene, a type of plastic designed to increase range of motion, improve joint stability and reduce the risk of wear.
Stryker also sells numerous femoral components, including press-fit stems, cemented stems, and revision components. Its press-fit stems include models from the Accolade and Secure-Fit product lines, and its revision components include products from the Restoration product line.
A 2008 study that analyzed 105 patients who received Secure-Fit hip implants concluded that the stems “performed exceedingly well” during the 5- and 10-year follow-up period. Four patients who had an elevated rim line developed osteolysis, a complication characterized by a loss of bone tissue.
Since 2012, there have been numerous complaints filed against the Stryker Corporation, one of the largest orthopedic and medical device manufacturers. Many patients who have received Stryker hip implants have complained of adverse side effects and extensive pain. When a company manufactures a medical device, they are responsible for presenting safe products, especially when someone's health is at stake.
Stryker's device has been known to cause severe injury and blood poisoning. Some other common side effects include metallosis, necrosis, osteolysis, heterotopic ossification, and periprosthetic fractures which we will discuss in-depth below.
Metallosis is a type of metal poisoning that can occur because of the metal-on-metal hip implant. Because the implant parts rub against each other, it leaves metal debris in the bloodstream that can negatively affect your body. Metallosis can cause nerve problems, impaired vision, groin pain, thyroid problems, and more.
Necrosis refers to the tissue and bone death that can occur because of the hip implant. With the implant, the cap covering the femoral ball can reduce the level of blood that reaches the surrounding tissue and bone. If these do not receive enough oxygen from the blood, it can lead to tissue and bone death.
Osteolysis is bone loss caused by metal debris in the body. The Stryker hip implants have a metal stem and neck that rub together, causing metal debris to form in the body. This bone loss can cause the implant to loosen and cause the risk of bone fractures.
During the heterotopic ossification process, soft tissue calcifies, causing the bone to form outside of the skeleton. Usually, in hip implants, the muscle around the device stiffens, leading to pain and immobility.
Periprosthetic fractures occur around the implant device. Because of causes such as metal poisoning, pressure, and medication, the bone around the device weakens, thus leading to fractures. These cracks can cause the implant to not work properly.
Hip Implant Surgery
- Hip implant surgery is often performed due to aging, injuries, and one of the most increasing factors, obesity.
- The surgery involves replacing the worn-out bone and cartilage lining the hip joint with new implants that are made of materials such as ceramic, metal, and plastic.
How Does It Work?
- During the surgery, the damaged cartilage surfaces at the ends of the femur and tibia are removed along with a small amount of underlying bone. The implants are then placed in the cavity, which recreates the surface of the joint.
- The procedure helps relieve pain caused by joint trauma or degenerative disease like osteoarthritis and restores mobility, making walking easier.
Types Of Hip Implants:
Currently, there are four types of total hip replacement devices available with different bearing surfaces:
- Metal-on-Polyethylene: The ball is made up of metal, and the socket is made of plastic (polyethylene) or has a plastic lining.
- Ceramic-on-Polyethylene: The ball is made up of ceramic, and the socket is made of plastic (polyethylene) or has a plastic lining.
- Ceramic-on-Ceramic: The ball is made up of ceramic, and the socket has a ceramic lining.
- Ceramic-on-Metal: The ball is made up of ceramic, and the socket has a metal lining.
Products From Stryker:
- Mobile Bearing Hip System
- Trident Acetabular Shell
- Trident Alumina Ceramic Bearing
- Trident Tritanium Acetabular Shell
- Trident II Tritanium Acetabular Shell
- Tritanium Acetabular Shell
- Trident Polyethylene Bearings
Serious Alleged Injuries May Include:
- Metallosis (The Build-Up Of Metal Ions In The Body)
- Inflammation And Swelling
- Limited Mobility
- Bone, Joint, Muscle Or Neurological Damage
- Necrosis-Tissue And Bone Death Due To Metallosis Toxicity
- Popping, Crunching Or Other Noises From The Hip Caused By Movement
- Fractures Of One Or More Components
FDA Safety Warnings:
Hundreds of adverse event reports were received by the FDA in 2012 involving the implants. This was followed by a voluntary recall by the manufacturer and then by the release of safety warnings by the FDA.
Legal Updates:
Hip recipients who were injured by Stryker's Rejuvenate and ABG II neck and stem implants filed lawsuits against Stryker Orthopaedics. Suits allege that Stryker designed faulty devices, failed to adequately test them in humans before marketing, failed to warn the public and medical community, and concealed information about the risks of adverse events caused by the devices.
In November 2014, Stryker agreed to settle several thousands of claims for $1.4 billion. This settlement encompassed cases in the New Jersey MCL and the Minnesota MDL and all the claimants who had revision surgery before Nov. 3, 2014. On the same lines, compensations were to be provided to patients operated on before Dec 19, 2016.
Lawsuits nearing 5,000 had been filed concerning both Stryker hip models since the 2012 recall. Two litigations had been set up due to the high number of claims: the multidistrict litigation (MDL 2441 before Judge Donovan W. Frank) in the U.S. District Court in the District of Minnesota, and state-level multi-county litigation (MCL) before Judge Brian R. Martinotti in Bergen County, New Jersey (NJ).
In November 2014, Stryker agreed to settle several thousand claims for $1.4 billion. This settlement encompassed the New Jersey MCL and the Minnesota MDL cases, and for all claimants who had revision surgery before Nov. 3, 2014. Stryker Hip recall settlement values were announced at up to $300K per hip. It was reached after four months of negotiations with Stryker mediated by retired U.S. Magistrate Judge Diane Welsh and had no cap on the fund. On the same lines, compensation was provided to patients operated on before Dec. 19, 2016.
In April 2017, MDL No. 2768 (In re Stryker LFIT V40 Femoral Head Products Liability Litigation) was formed to be presided by U.S. District Judge Indira Talwani in the United States District Court for the District of Massachusetts. The first bellwether is to commence on September 16, 2019.
Important Legal Proceedings, Verdicts, & Settlement:
November 2019: Stryker issues a safety notice concerning its STAR Total Ankle Replacements distributed before August 2014. The notice warns patients and doctors about the risks associated with the ankle replacements, which could result in significant pain, loss of mobility, and require revision or replacement surgery.
November 2018: Stryker Incorporation announces a confidential, private settlement before the MDL Judge Indira Talwani in Boston over cases linked to both recalled and unrecalled components of Stryker LFIT Cobalt Chromium V40 Femoral Heads used in hip correction surgeries.
June 2018: A safety alert by hip implant manufacturer Stryker revealed that certain sizes of their LFIT V40 Hip implant may rupture.
January 2018: The United States District Court for the District of Massachusetts overseeing Stryker Hip Replacement lawsuits extends the date for Plaintiff Fact Sheets submission from January 12 to January 26, 2018.
July 2017: Three plaintiffs who filed lawsuits against Surgi-Care, a Massachusetts distributor of the Stryker hip components, wherein they suffered hip replacement complications following implantation of Stryker's Accolade TMZF Hip Stem and LFIT Anatomic V40 Femoral Head put forth a motion to remand their claims to the Massachusetts state court.
Evidence:
- Indication Of Usage In Medical And Pharmacy Records
- Implant Sticker And Relevant Details Like Product Code, Manufacturer, Lot Number
- Follow Up Complications And Their Treatment After Stryker Hip Implant Insertion
- Revision Surgeries And Implants
Medical Record Review and claim validation of Stryker Hip Implant case should take approximately 3 hours in most instances; however, this approximation may vary in cases based on the volume of records.