Textured Breast Implants Associated With Cancer Recurrence
Textured Breast Implants Associated With Cancer Recurrence
Introduction
Last week, a report was published in the medical journal JAMA Surgery, where South Korean researchers suggested an association between the use of textured breast implants and an increased risk of breast cancer relapse.
The cohort study over the question of whether the surface type of the breast implant alters the outcome of the original breast cancer was carried at Samsung Medical Center in Seoul, South Korea.
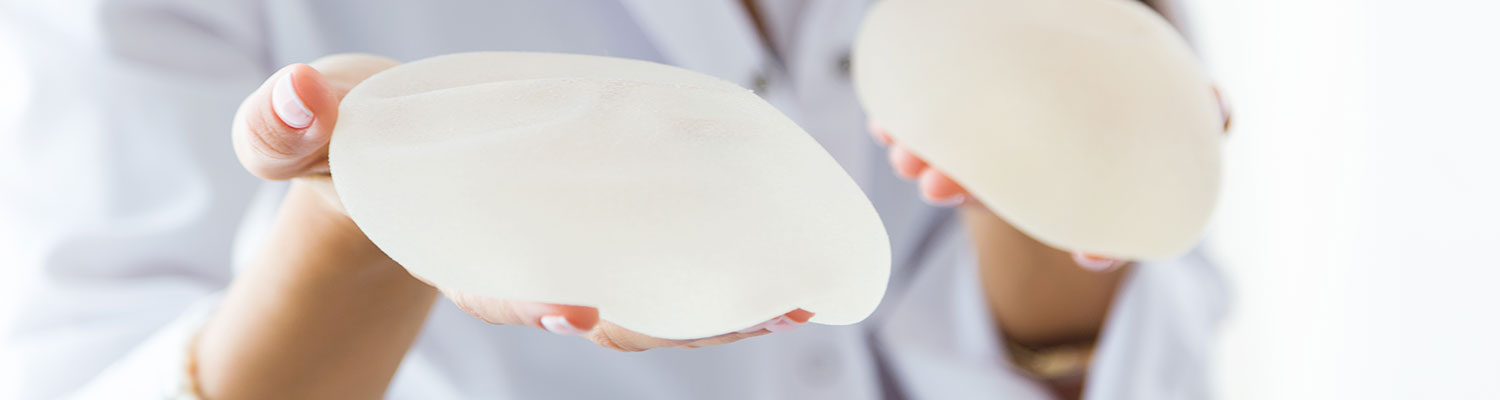
The study included 650 women, identified from a prospectively maintained database, who underwent 687 breast implant procedures, including tissue expanders and breast reconstruction, from January 1, 2011, to December 31, 2016. They were further categorized into two groups according to the surface type of implant, i.e., smooth or textured implants. Of the 687 cases, 274 (39.9%) received a smooth implant, and 413 (60.1%) received a textured implant.
The patients were followed up for at least two years after the surgery, and an analysis was performed from February 15, 2020, to March 5, 2020. The analysis revealed that women who had textured breast implants faced three times the risk of relapse and significantly lower disease-free survival rate as compared to the ones who received implants with smooth surfaces.
The researchers concluded that the use of textured implants in reconstruction appears to be associated with the recurrence of breast cancer, and further investigation is required to verify these results.
In August, the Food and Drug Administration (FDA) released a report indicating that the number of breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) cases increased by nearly 28% in the second half of last year. The report showed a total of 733 BIA-ALCL diagnoses, along with 36 patient deaths globally. Out of the known cases where the manufacturer has been identified, 620 are linked to breast implants sold by Allergan, Inc., and in 47 cases, the manufacturers were unknown. Only 16 manufacturers were identified, of the 36 deaths, and 15 of those involved Allergan implants.
Currently, Allergan faces at least 150 product liability lawsuits and class action lawsuits over its breast implants, each claiming that the textured design was unreasonably dangerous and defective, and the manufacturer knew that it increased the risk of BIA-ALCL yet failed to warn about those risks.




