Whittaker Clark & Daniels, Johnson & Johnson Talcum Powder supplier, submitted an appeal to dismiss an asbestos-in-talc lawsuit filed against them, on the grounds that the company has limited contacts with Florida, where the claim was filed. The supplier
Whittaker Clark & Daniels, Johnson & Johnson Talcum Powder supplier, submitted an appeal to dismiss an asbestos-in-talc lawsuit filed against them, on the grounds that the company has limited contacts with Florida, where the claim was filed. The supplier
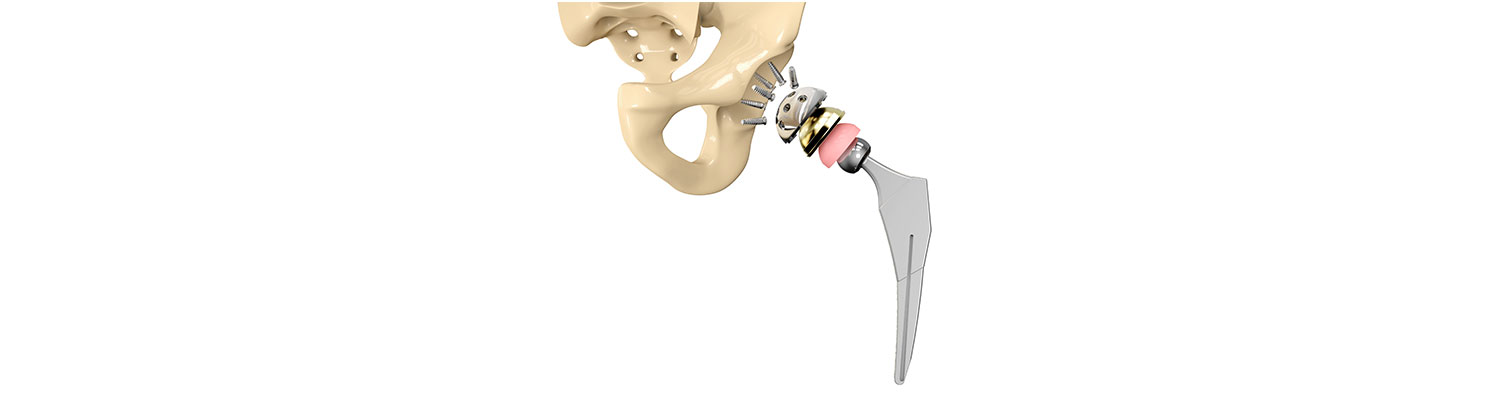
Introduction
A safety alert by hip implant manufacturer Stryker reveals that certain sizes of their LFIT V40 Hip implant may rupture. The company received a higher than expected number of charges stating that the femoral head broke free of the stem that connects it to the thigh bone requiring revision surgery.
Stryker Orthopaedics is known for the development and manufacture of specialty surgical products and other medical supplies for health care around the world. The company has manufactured scores of medical devices and joint replacement products, most of which have been successful; however, some of the hip replacement devices have caused serious adverse events, injuring hundreds of patients.
The Stryker Rejuvenate Modular-Neck Stem and the ABG Modular-Neck Stem hip replacement devices were approved by the Food and Drug Administration (FDA) in 2008 and 2009. These devices are not typical "metal-on-metal" (MoM) as the necks are built of Cobalt and Chromium and the neck stems are layered with Titanium. Though the intention was to make it corrosion resistant, the two metals rubbed on each other at the connection and released toxic metal ions and debris. Stryker permanently recalled the Rejuvenate Modular and ABG II Modular-Neck Hip Stems in 2012 and stopped all global sales and production of these components. Another hip stem model, the Accolade TMZF, also caused problems and was recalled in 2009, 2011, and 2013.
Stryker identified eight sizes of LFIT V40 femoral heads that may cause complications and informed health centers to alert the patients. The FDA will decide in a month or two whether to consider a recall for the product or not. An earlier Class II recall by FDA in 2016 affected more than 42,500 Stryker hip replacement products.
Cases against Stryker hip replacement systems are consolidated as a part of multidistrict litigation, MDL No. 2768 ( In Re: Stryker LFIT V40 Femoral Head Products Liability Litigation) in the United States District Court for the District of Massachusetts before U.S. District Judge Indira Talwani.




