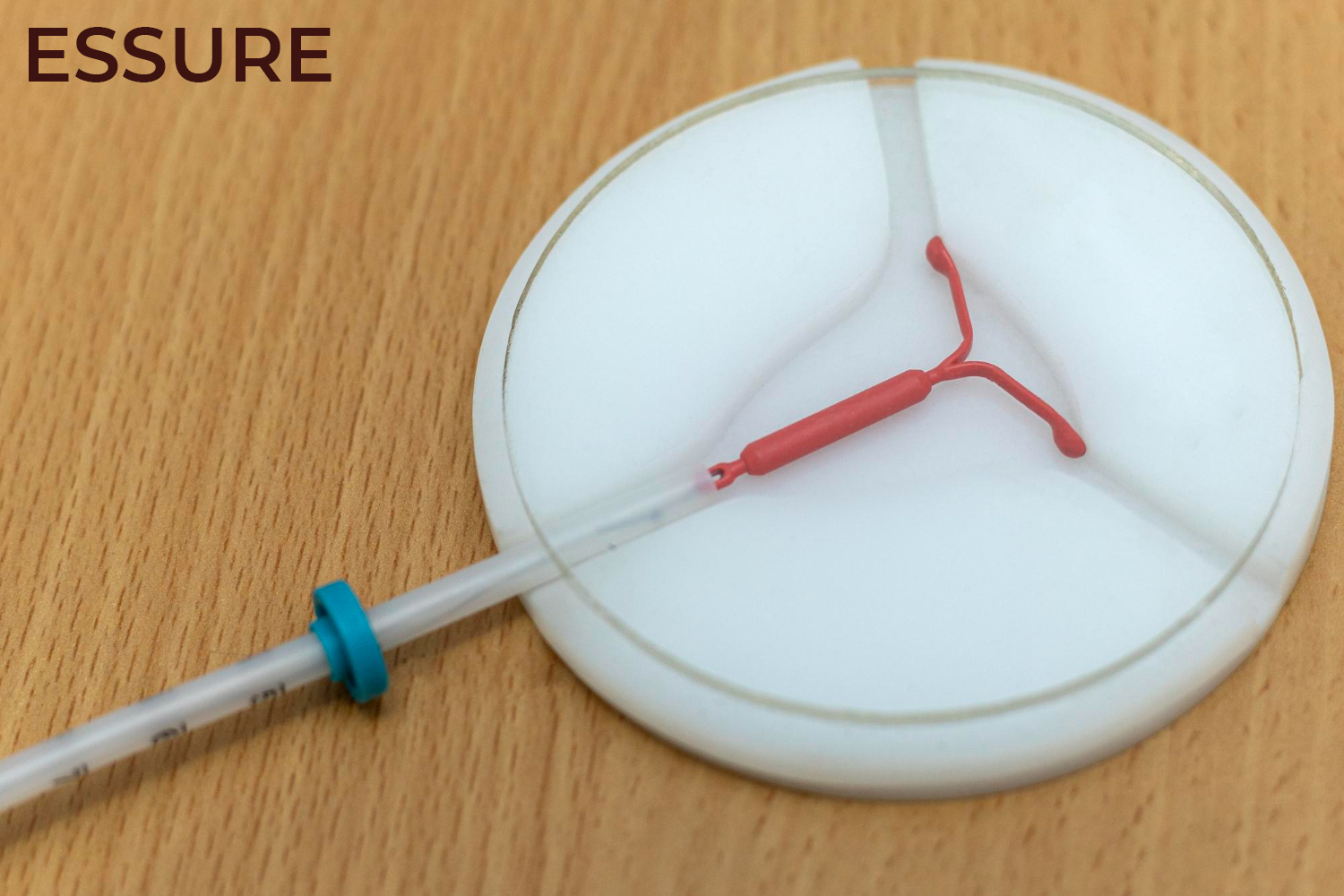
Conceptus Inc., a subsidiary of Bayer AG, developed Essure which is a Class III device for permanent surgical sterilization in women. Essure was approved by the Food and Drug Administration (FDA) on November 4, 2002. The device has two metal coils made from polyester fibers, nickel-titanium, stainless steel, and solder which are placed in fallopian tubes to induce fibrosis and block the fallopian tubes to prevent fertilization. Since 2002, it is estimated by Bayer that 750,000 women have received the Essure IUD.
Advantages of Essure as marketed by Bayer
Less Anaesthesia
Less Time Procedure
Less Invasive
Cheaper
Highly Effective
Essure's warning label had noted that the device's nickel can result in allergic reactions, such as itching and hives but in reality, women were found to suffer permanent injuries including autoimmune diseases, perforated organs, and severe pelvic pain. From November 4, 2002, through May 31, 2015, around 5,093 reports of problems connected to Essure were filed with FDA.
While the device's manufacturer claims Essure is safe and effective, thousands of women disagree. They say the metal coils caused them injuries such as perforation of the fallopian tube, neurological damage, and severe pain. In some cases, women died from complications.
Serious Alleged Injuries May Include:
- Severe Abdominal Pain And Bloating
- Severe Pelvic Pain
- Device Displacement
- Device Breakage
- Heavy Menstrual Periods
- Weight Fluctuations
- Allergies To Nickel
- Chances Of Ectopic Pregnancy
- Permanent Removal Of Uterus
- Perforation Of The Uterus Or Fallopian Tubes
- Hair Loss
- Fetal Death
- Chronic Pain
FDA Safety Warnings:
- In September 2015, the FDA held a meeting to examine the safety of the product after receiving thousands of adverse event reports.
- On October 31, 2016, the FDA issued final guidance, “Labeling for Permanent Hysteroscopically-Placed Tubal Implants Intended for Sterilization” after carefully considering public comments.
- On November 11, 2016, FDA announced label changes adding a boxed warning and a Patient Decision Checklist. This was aimed to support patient counseling and understanding of risks and benefits associated with Essure, as well as what to expect during and after the Essure procedure.
- On April 9, 2018, FDA restricted the sale of Essure device to only those doctors and healthcare facilities who use the FDA-approved “Patient-Doctor Discussion Checklist – Acceptance of Risk and Informed Decision Acknowledgement”. In July 2018, Bayer announced Essure would be wiped out of the market by December 31, 2018.
Legal Updates:
Defendant: Bayer AG
Defense Law Firm: Sidley Austin LLP
Defendant Lead and Liaison Counsel: Alycia A. Degen - Sidley Austin LLP
Allegations: More than 17,000 lawsuits have been filed against Bayer Healthcare blaming the company for negligent conduct, failure to warn the public about the health risks, breach of implied and express warranty, fraud, willfully hiding the health hazards of Essure, misrepresenting the safety and effectiveness of the device and improperly training health care providers on how to use Essure.
Plaintiffs' Steering Committee:
The California Court appointed the below-listed counsel as Plaintiffs' Steering Committee on February 16, 2017, before Honorable Judge Winifred Y. Smith for Essure Product Cases Judicial Council Coordination Proceeding (JCCP) No. 4887:
Sindhu Daniel - Baron & Budd
Will Moody - The Moody Law Firm Inc.
Martin Schmidt - Schmidt National Law Group
Karen Schroeder - Schroeder Law Office PLLC
Laura Yaeger - Morgan & Morgan
Yvonne Flaherty - Lockridge Grindal Nauen PLLP
Jennifer Lenze - Lenze Kamerrer Moss PC
Sean Jez - FLeming Nolen Jez LLP
Fred Hagans - Hagans Montgomery & Rustay PC
Lori Andrus - Andrus Anderson LLP
Steven Rotman - Hausfeld LLP
Justin Browne - Janet, Jenner & Suggs LLC
Lawsuit Status:
California Essure lawsuits are under Judicial Council Coordinated Proceedings (JCCP), which will facilitate efficient adjudication and coordinate efforts on behalf of the plaintiffs, and presided by Superior Court Judge Winifred Y. Smith. The Plaintiff Fact Sheet (PFS) for lawsuits filed under JCCP has been released by the Court.
In 2017, the company reports quoted a loss of about $413 million in revenue in 2016 because of Essure lawsuits and was facing about 3,700 lawsuits.
Timeline:
Verdicts & Settlement: To date, there have been no Essure lawsuit settlements.
November 2002: FDA approved Essure as a Class III medical device for permanent birth control.
June 2008: Bayer was notified by the FDA for marketing Essure implants in an unlicensed factory till 2005 and failed to document the procedures appropriately.
January 2011: FDA warned Bayer for violating the premarket approval and making design changes with seeking additional permission.
February 2016: Based on adverse event reports from women who were affected by the birth control device, FDA ordered Bayer to conduct a new clinical trial to analyze the risks involved in using the device.
August 2016: Presiding Judge Winifred Y. Smith of the Alameda County Superior Court allowed consolidating all Essure lawsuits filed under Judicial Council Coordination Proceedings (JCCP) in August 2016. This was followed by appointments to the Plaintiff's Steering Committee (PSC) in February 2017 to initiate, coordinate, and conduct pre-trial case discovery.
November 2016: The FDA ordered Bayer to update their Essure's product label with a black box warning indicating the risks of internal organ perforation, chronic abdominal pain, and allergy associated with the use of the birth control method.
February 2017: Formation of Essure Product Cases and Coordinated Actions, Judicial Council Coordination Proceeding No. 4887 was followed by appointments to the Plaintiff's Steering Committee (PSC) in February 2017, to initiate, coordinate, and conduct all pre-trial discovery on behalf of the plaintiffs and also provide input to the Executive Committee.
April 9, 2018: FDA restricted the sale of Essure device to only those doctors and healthcare facilities who use the FDA-approved “Patient-Doctor Discussion Checklist – Acceptance of Risk and Informed Decision Acknowledgement”.
July 2018: Reports from CNN revealed that Bayer influenced several doctors by paying about $2.5 million to suggest Essure as a birth control method to patients.
July 2018: Bayer announced Essure would be wiped out of the market by December 31, 2018.
Evidence:
- Usage Of Essure Device In Operative Records.
- Product Identification (Implant Sticker) In The Medical Records.
- Proof Of Injury In Medical Records.
- Treatment Provided For The Injuries.
Medical Record Review and claim validation of Essure case should take approximately 2 hours in most instances; however, this approximation may vary in cases based on the volume of records.