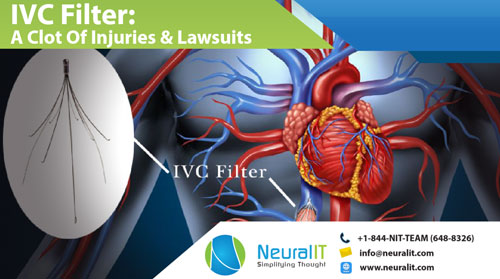
An Inferior vena cava filter (IVC filter), earlier popularly known as Greenfield filter, is a medical device implanted in the inferior vena cava just below the kidneys to capture blood clots, preventing them from reaching the heart and lungs, thereby, safeguarding against life-threatening pulmonary emboli (PE). IVC filters were cleared for use through the 510(k) process since 1976 however, in 2010 the FDA issued a device safety communication after reviewing more than 900 adverse events related to the devices over a period of five-years C.R. Bard, Inc. and Bard Peripheral Vascular, Inc. (collectively, Bard) and Cook Incorporated, Cook Medical LLC, and William Cook Europe ApS (collectively, Cook) are the main manufacturers of retrievable IVC filters. Other manufacturers include Argon Medical Devices, Cordis Corporation, Rex Medical, Johnson & Johnson, ALN, B. Braun Medical, and Rafael Medical.
The IVC Filter deployment is done by interventional radiologists or vascular surgeons. It is generally recommended to patients for whom anticoagulation therapy is contraindicated or ineffective.
Here is our analysis of the
IVC Filter: A Clot Of Injuries & Lawsuits
Serious Alleged Injuries May Include:
- Device-Associated Morbidity
- Device Migration
- Recurrent DVT/PE
- Filter Fracture
- Thrombotic Complications
- Insertion-Site Thrombosis
- Perforation Of The Vena Cava
- Filter Embolization
- Vena Cava Thrombosis
FDA Safety Warnings:
- The FDA received more than 6,000 adverse event reports related to inferior vena cava filter side effects.
- In 2005, the Greenfield Vena Cava Filter produced by Boston Scientific was recalled by the FDA, which was terminated in November 2006.
- On August 9, 2010, an Advisory Letter was released by the FDA, recommending health care providers to consider removing the temporary filter as soon as protection from a PE is no longer needed.
- In 2013, the FDA announced a recall mentioning a labeling correction of a retrievable IVC filter manufactured by Cordis Corporation.
- On May 6, 2014, the FDA recommended the removal of temporary IVC filters within 29-54 days through the release of another Advisory letter.
- In 2015, the FDA sent a warning letter to C.R. Bard Inc. for violations and failure to warn of its IVC filter devices.
Legal Updates:
IVC Filter lawsuits came under scanner for injuries caused to internal organs of the patients as the device was prone to break and migrate to internal organs. Device fracture caused clot formation around the device causing serious complications among several individuals.
Class action suits have also been filed against the device manufacturers accusing them of negligence, concealment, and misrepresentation of data concerning the safety of its filters.
Defendants: C.R. Bard, Cook Medical, and Boston Scientific
Allegations: As of October 2018, 9,000 IVC filter lawsuits have been filed in the federal court over allegations of design and manufacturing defects, failure to adequately warn consumers of the device-related health hazards, breach of implied warranty on the medical device, negligent conduct on the part of the manufacturing and/or marketing company.
Devices Reported To Be Faulty:
- Recovery Filter
- G2 Filter
- G2 Express
- Celect Filter
- Günther Tulip
- Greenfield Filter
Defense Law Firm for Cook Medical LLC: Faegre Baker Daniels LLP
Defendants’ Lead Counsel
Mr. Richard North of Nelson - Mullins Riley & Scarborough, LLP, in Atlanta, Georgia
(MDL No. 2641; In Re: Bard IVC Filters Products Liability Litigation)
Plaintiffs’ Steering Committee
- Russell W.Budd - Baron & Budd, P.C.
- William B. Curtis - Curtis Law Group
- Brian Keith Jackson - Riley & Jackson, P.C.
- Christopher T. Kirchner - Provost Umphrey Law Firm, L.L.P.
- John “Scotty” MacLean - MacLean Law Firm, P.C.
- Howard L. Nations - The Nations Law Firm
- Gregory D. Rueb - Rueb & Motta, PLC
- Laura E. Smith - Heaviside Reed Zaic
- Paul L. Stoller - Gallagher & Kennedy, P.A.
- David C. DeGreeff - Wagstaff & Cartmell LLP
- Willard James Moody, Jr. - The Moody Law Firm, Inc.
- Jonathan M. Sedge - Weitz & Luxenberg, PC
- Matthew D. Schultz - Levin, Papantonio, Thomas, Mitchell, Rafferty, & Proctor, P.A.
- Robert M. Hammers - Jr., Schneider Hammers LLC
- Charles S. Siegel - Waters Kraus & Paul
Lawsuit Status:
Two MDLs have been formed for consolidated pretrial proceedings.
MDL No. 2570
(IN-RE: Cook Medical, Inc., IVC Filters Marketing, Sales Practices, and Products Liability Litigation)
- Formed in October 2014.
- The plaintiffs allege defect, misrepresentation in marketing, and failure to warn doctors and patients.
- The cases are coordinated in the Southern District of Indiana and assigned to Judge Richard L. Young and Magistrate Judge Tim A. Baker.
MDL No.: 2641
(IN RE: Bard IVC Filters Products Liability Litigation)
- Formed in August 2015
- The plaintiffs allege negligence to warn patients and doctors about the device defect, misrepresentation in marketing, and device fracture in their complaints.
- The cases are coordinated in the U.S. District Court District of Arizona presided by U.S. Federal Judge David G. Campbell. As of September 2018, 3,800 lawsuits are pending against Bard in the state of Arizona.
Class action suits have also been filed against Bard accusing the company of negligence, concealment, and misrepresentation of data concerning the safety of its filters.
Important Verdicts and Settlements
- November 2017: Cook Medical won the first bellwether trial over the significant injuries caused by their Celect IVC filter brand on November 9, 2017. The Indiana jury failed to find the company at fault for the plaintiffs injuries that included migration and perforation of the vena cava.
- March 2018: Plaintiff Sherr-Una Booker was awarded $3.6 million by an Arizona jury on March 30, 2018, as C.R. Bard was found liable for failing to warn doctors and public about their Bard G2 blood filter. This was the first bellwether trial against Bard.
- March 2018: The second bellwether trial against Cook in the U.S. District Court for the Southern District of Indiana was dismissed on April 30, 2018, as the statute of limitations expired on those claims.
- May 2018: A Houston firefighter, Jeff Pavlock, was awarded more than $1.2 million by a Texas jury for the lawsuit filed against Cook Medical LLC involving a Celect IVC filter. The lawsuit stated the filter tilted and pierced into his tissues causing an internal injury, requiring revision surgery. The allegations include failure to warn the performing surgeon about the possible adverse effects of the defectively designed filter. This case was not part of the federal bellwether.
- June 2018: The second bellwether trial involving C.R. Bard's Eclipse IVC Filter ended in the defendant's favor on June 1, 2018, as the Arizona jury found that adequate warning was provided to doctors regarding the possible adverse effects of the IVC filter. Plaintiff Doris Jones of Georgia accused the defective design of the deep vein thrombosis (DVT) filter of her arm pain and headaches.
- October 2018: C.R. Bard prevailed in the fourth bellwether trial Hyde v. Bard after the third bellwether was dismissed as the statute of limitations expired on those claims.
- December 2018: On December 5, Judge Richard L. Young granted summary judgment to Cook Medical Inc. on failure-to-warn claims in an IVC filter bellwether case filed by Georgia resident Tonya Brand.
- February 2019: On February 1, 2019, an Indiana federal jury awarded $3 million in the third bellwether case involving Cook Medical's Inferior Vena Cava (IVC) filter injuries, finding that the company's product was the reason for a woman's complications.
Evidence:
- Usage Of IVC Filters In Operative Records.
- Indications For The Usage Of IVC Filter From Medical Records
- Evidence Of Injury In Follow Up Medical Records
Medical Record Review and claim validation of IVC Filter case should take approximately 6 hours in most instances; however, this approximation may vary in cases based on the volume of records.