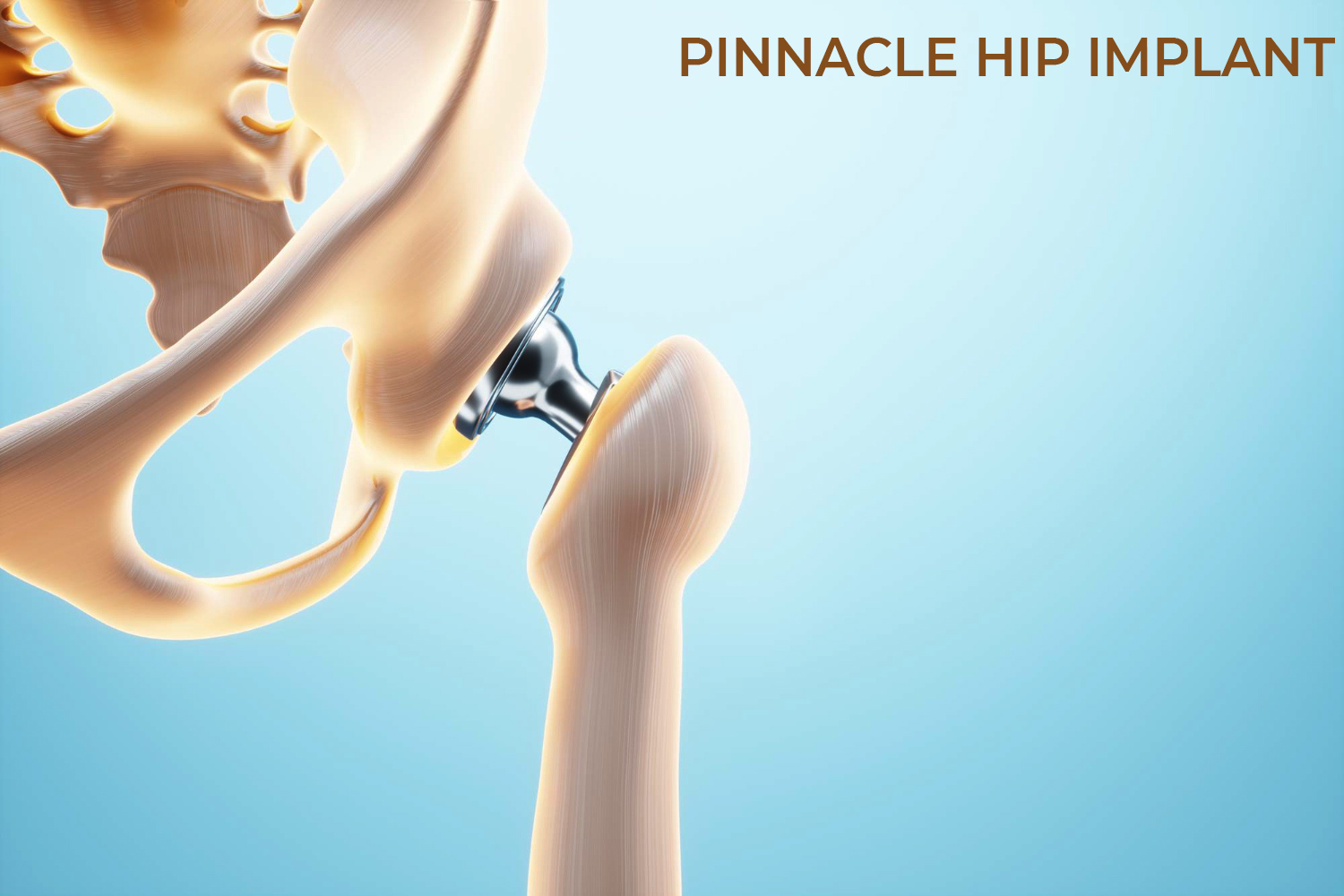
DePuy Synthes, acquired by Johnson & Johnson (J&J) in 1998 is one of the orthopedic franchises involved in manufacturing, designing, marketing products for repairing, reconstructing, correcting, various bone fractures and joint deformities. DePuy provides following product for Hip Replacement Surgery: DePuy Articular Surface Replacement (ASR) Hip Resurfacing System, ASR XL Acetabular System for total hip replacement and Pinnacle hip replacement system being their top hip replacement products.
The DePuy Pinnacle was initially approved by the U.S. Food and Drug Administration (FDA) in 2000. The 510(k) approval was based on similarities to the other implant and hence did not undergo the usual safety tests. ASR and ASR XL have been recalled in 2010 for its failure, whereas the sales of metal Ultamet liner ended in May 2013. However, the sales of Pinnacle hips made from other materials were not affected.
DePuy stated the reason for failure as component loosening, infection, dislocation, component malalignment, fracture of the bone, metal sensitivity and pain. Additional complications include bone staining, necrosis, swelling, increased metal ion levels in the blood, nerve damage, tissue damage and/or muscle damage.
In the year 2017 when Johnson & Johnson (J&J) and its subsidiary company DePuy were busy settling as many as 11,000 DePuy ASR hip implant lawsuits. The orthopedic device manufacturer was confident about their product safety for hip implant surgery. Johnson & Johnson refused to recall the Pinnacle hip implant, despite the more than 9,000 claims from Pinnacle victims. At that time, the Pinnacle hip lawsuits alleged the Pinnacle was defectively designed.
Plaintiffs claimed when they engaged in any level of activity, the metal parts of the implant would come into contact with one another, rubbing together and causing cobalt and chromium ions to imbed into surrounding tissues or to enter the bloodstream.
More than one million patients have received Pinnacle Hip Solutions cups, according to DePuy. The devices are implanted into the pelvis during partial or total hip replacement surgeries to relieve pain or increase mobility. Today, the Pinnacle cups come in ceramic-on-polyethylene and metal-on-polyethylene options depending on patient
In 2010, DePuy recalled its ASR XL Acetabular System and ASR Hip Resurfacing System because a large number of patients required revision surgery after receiving the implants. The company also stopped selling the all-metal versions of the Pinnacle system of implants because large numbers of consumers claimed the devices were faulty and led to a type of metal poisoning known as metallosis.
Plaintiffs in the Pinnacle cases claim that J & J and DePuy were well-aware of the flaws in the Pinnacle device, however rather than issuing a recall of the device, marketing tactics were increased. The companies claimed the Pinnacle was the best hip implant option for younger patients with an active lifestyle, yet some patients found themselves unable to even walk without painless than a year after the device was implanted.
After hip implantation, severe inflammation in the hip and thigh areas can be caused which include chronic pain in the thigh, hip, and groin areas, bone and tissue deterioration, and various other physical damages.
When the cobalt and chromium ions entered the bloodstream during surgery, the effects can be even more damaging which include severe headaches, visual disturbances, memory loss, vertigo, hearing loss, DNA disruption, and gastrointestinal disorders.
Johnson & Johnson reversed its prior position on the Pinnacle hip lawsuits in the year 2019, agreeing to settle the bulk of consumers lawsuits which alleged the Pinnacle hips were defective and that J & J and DePuy misled patients about the danger of the implants. While DePuy marketed the Pinnacle implants as having a five-year rate of more than 90 percent, European health regulators found that number to be about 5 percent. This settlement agreement came two weeks after J&J agreed to pay $120 million to resolve claims of deceptive marketing to state attorneys general. In an earlier Pinnacle settlement, Johnson & Johnson paid about $125,000 per case to resolve roughly one-third of the more than 10,000 Pinnacle implant claims.
An Insider Look: Hip Implant Litigation from Neural IT
Serious Alleged Injuries May Include:
- Premature Loosening
- Severe Pain
- Inflammation And Swelling
- Limited Mobility
- Infection
- Bone, Joint, Muscle Or Neurological Damage
- Instability When Standing Or Putting Weight On The Pinnacle Hip Implant
FDA Safety Warnings:
February 2016
According to a final order published by FDA, all manufacturers of metal-on-metal total hip implants were required to stop marketing their devices and submit premarket approval applications effective May 2016. The approval was based on sufficient valid scientific evidence to reasonably assure that the device is safe and effective for use. There are no FDA-approved MoM total hip replacement devices marketed for use in the U.S.
May 2011
The FDA issued 522 orders instructing all five U.S. manufacturers of MoM total hip replacement devices on the market at that time, to conduct a postmarket surveillance study of their devices.
Legal Updates:
Defendants: DePuy Orthopaedics, Inc.; DePuy Products, Inc.; DePuy International, Limited; Johnson & Johnson; and Johnson & Johnson Services, Inc.
Defendant Law Firm: E. Leon Carter of Carter Arnett PLLC, Steven W. Quattlebaum of Quattlebaum Grooms & Tull PLLC, Tracie J. Renfroe of King & Spalding LLP, and John H. Beisner, Stephen J. Harburg and Jessica Davidson Miller of Skadden Arps Slate Meagher & Flom LLP.
Allegations: Allegations include that the company sold defective artificial hips and misled patients about their dangers.
Plaintiff Steering Committee
Seventeen attorneys from across the country make up the plaintiffs steering committee:
Franklin D. Azar of Franklin D. Azar & Associates, P.C.
Thomas P. Cartmell of Wagstaff & Cartmell, LLP
John R. Climaco of Climaco, Wilcox, Peca, Tarantino & Garofoli Co., LPA
Ben Gordon of Levin Papantonio Thomas Mitchell Rafferty & Proctor, P.A.
Lawrence J. Gornick of Levin, Simes, Kaiser & Gornick LLP
Jeff Grand of Bernstein Liebhard, LLP
Marc D. Grossman of The Sanders Firm
Steve Harrison of Harrison, Davis, Steakley & Morrison, PC
Don Migliori of Motley Rice
Trent Miracle of The Simmons Firm
Jerold S. Parker of Parker, Waichman & Alonso
John M. Restaino of The Restaino Law Firm
Peter Samberg of Weitz Luxenburg
Jane Lamberti Sams of The Cochran Law Firm
Christopher A. Seeger of Seeger Weiss LLP
Hunter Shkolnik of Napoli BernRipka Shkolnik, LLP
Navan Ward of Beasley Allen Law Firm
Lawsuit Status:
Nearly 11,000 lawsuits have been filed against DePuy over ASR and Pinnacle hip implant defects. The Pinnacle hip lawsuits are consolidated before U.S. District Judge Ed Kinkeade since 2011 (MDL 3:11-MD-02244, In Re: DePuy Orthopaedics, Inc., Pinnacle Hip Implant Products Liability Litigation)
Important Verdicts & Settlements:
May 2019: J&J agrees to pay $1 billion to resolve claims involving personal injury linked to the usage of a metal-on-metal version of DePuy Orthopaedics.
January 2019: J&J and its DePuy Orthopaedics unit agree to pay $120 million to resolve defective hip implant claims filed by 46 states.
December 2018: J&J agrees to pay more than $400 million to settle some product liability cases concerning Pinnacle hip-replacement devices.
December 2018: The federal judge in Texas, overseeing J&J's Pinnacle Hip litigation, indicates that nearly 3,300 of 10,000 artificial hip implant cases filed were in the process of settlement.
October 2018: A formula was devised by an Indian Government panel of experts asking J&J to pay ₹1.2 crore to each Indian patients who suffered severe complications allegedly due to its faulty hip implants.
September 2018: The Indian Drug Regulator asked J&J to pay ₹20 lakh as interim compensation to each affected individual who suffered complications due to the company's defective hip implant, Articular Surface Replacement (ASR), manufactured by its subsidiary DePuy Orthopaedics.
November 2017: J&J and its subsidiary DePuy Orthopaedics were ordered to pay $247 million to a group of 6 plaintiffs by a Texas jury for the alleged injuries linked to their DePuy Pinnacle Hip Implant.
Evidence:
- Detailed Operative Reports
- Usage Of Pinnacle Hip Implant
- Implant Sticker And Relevant Details Like Product Code, Manufacturer, Lot Number
- Follow-Up Complications And Their Treatment After Pinnacle Hip Implant Insertion
Medical Record Review and claim validation of Pinnacle Hip Implant case should take approximately 3 hours in most instances; however, this approximation may vary in cases based on the volume of records.